Table of Contents=> Go Directly
LAW OF CONSERVATION
The law of conservation of mass or principle of mass conservation states that for any system closed to all transfers of matter and energy, the mass of the system must remain constant over tie, as system’s mass cannot change, so quantity cannot be added nor removed. Hence, the quantity of mass is conserved over time.
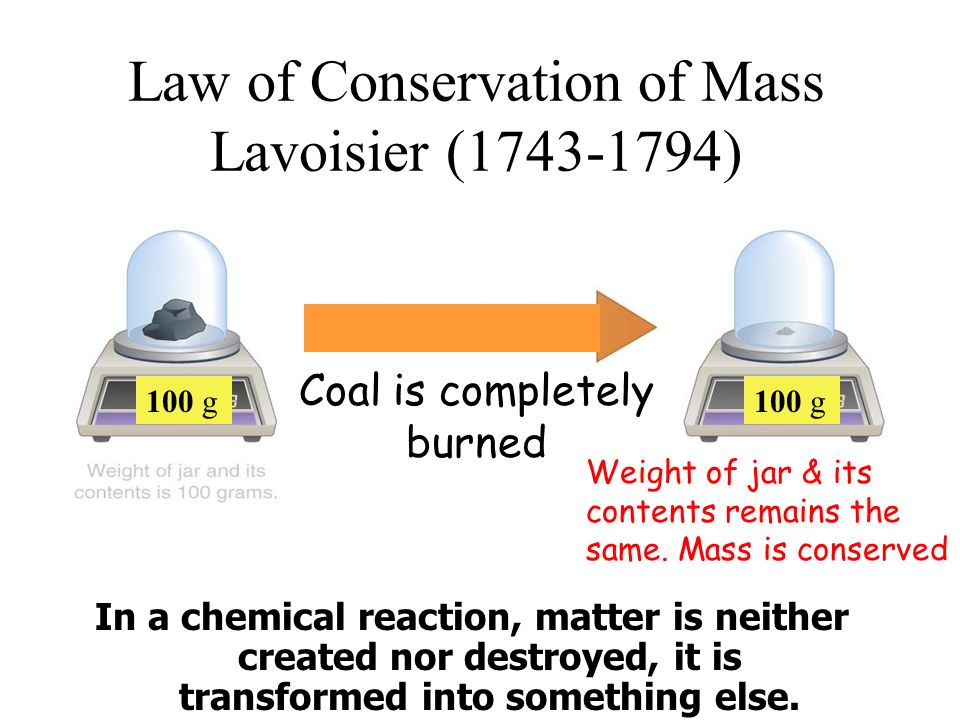
The law implies that mass can neither be created nor destroyed, although it may be rearranged in space, or the entities associated with it may be changed in form. For example, in chemical reactions, the mass of the chemical components before the reaction is equal to the mass of the components after the reaction. Thus, during any chemical reaction and low-energy thermodynamic processes in an isolated system, the total mass of the reactants, or starting materials, must be equal to the mass of the products.
Important Questions asked in Exams ( #3 )
1. Who first Gave the Law of Conservation of Mass ?
=>Lavoisier (1774 A.D)
2. Who First tasted the Law of Conservation of Mass ?
=>Landolt
3. Who First Gave the Law of Constant Properties ?
=>Proust (1799 A.D)
4. Who First Gave the Law of Multiple properties ?
=>Dalton (1800 A.D)
5. Who First Gave the Law of Reciprocal properties ?
=>Richter (1794 A.D)
6. Who First Gave the Law of Gaseous volumes ?
=>Gay-Lussac (1808 A.D)
7. What is Chemical name of Dry Skin ?
=>Carbon di oxide ( solid= co2 )
8. What is Chemical formula of Sugar ?
=>Sucrose ( C12H22O11 )
9. Who first Given the Atomic Theory ?
=>John’s Dalton
10. How to Calculate Atomic mass of Element in gram , Give Formula ?
=>The atomic mass of element in amu × 1.66×10-24 gram.
=>Ex=> Oxygen = 16.00 amu × 1.66×10-24 gram
= 2.656×10-23 gram .
ATOMIC THEORY=>
An atomic theory is a model developed to explain the properties and behaviors of atoms. As with any scientific theory, an atomic theory is based on scientific evidence available at any given time and serves to suggest future lines of research about atoms.
The concept of an atom can be traced to debates among Greek philosophers that took place around the sixth century B.C. One of the questions that interested these thinkers was the nature of matter. Is matter, they asked, continuous or discontinuous? That is, if you could break apart a piece of chalk as long as you wanted, would you ever reach some ultimate particle beyond which further division was impossible? Or could you keep up that process of division forever? A proponent of the ultimate particle concept was the philosopher Democritus (c. 470–c. 380 B.C. ), who named those particles atomos. In Greek, atomos means “indivisible.”
